Details of the Drug
General Information of Drug (ID: DM9HG1Y)
| Drug Name |
DCG IV
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Dcg-IV; DCG IV; 147782-19-2; CHEMBL284193; (1r,2r)-3-[(S)-Amino(Carboxy)methyl]cyclopropane-1,2-Dicarboxylic Acid; (1R,2R)-3-[(1S)-1-amino-2-hydroxy-2-oxoethyl]cyclopropane-1,2-dicarboxylic acid; DCGIV; AC1NSJRH; (2S,2'R,3'R)-2-(2',3'-dicarboxycyclopropyl)glycine; SCHEMBL658075; GTPL1377; 1,2-Cyclopropanedicarboxylicacid, 3-[(S)-aminocarboxymethyl]-, (1R,2R)-; CTK0H7426; MATPZHBYOVDBLI-JJYYJPOSSA-N; ZINC3995770; BDBM50034503; AKOS030230932; API0015797; B5039; CS-0021174; (2S,2''R,3''R)-2-(2'',3'')-dicarboxycyclopropylglycine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
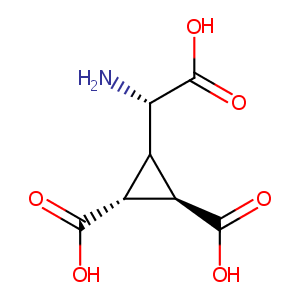 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 1 | Molecular Weight (mw) | 203.15 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -4.3 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 4 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 7 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
